Edmonton, Alberta, Canada, March 4, 2020 – PACYLEX PHARMACEUTICALS INC. (Pacylex), a pre-clinical pharmaceutical company, announces it raised over US$3.9M in a 2020 convertible note financing led by a group of Canadian angel investors from Edmonton. These funds will support advancing the Company’s first product, PCLX-001, to clinical trials anticipated to start later this year. PCLX-001 targets hematologic cancers and is a new, first-in-class therapeutic. Pacylex previously raised approximately US $2 million in convertible note financings in 2018 and 2019 from a mix of US and Canadian angel investors.
“Doing something for the first time is exciting. Getting to be the first to do something that has never been done before is exhilarating. We are grateful to our investors for joining us to support our next exhilarating clinical adventure” said Dr. Luc Berthiaume, CSO and co-founder of Pacylex, a University of Alberta spin-off company.
Board changes and development partnership.
In connection with the investment, Mark Huson, a professor of finance at the University of Alberta, joined the Pacylex board. Ryan Heit, a co-founder of Pacylex, retired from the board and will remain active as the Company’s chief operating officer.
Pacylex also announces a partnership with DavosPharma to oversee the preparation of clinical supplies and regulatory clearances to advance PCLX-001 through development for Phase I clinical trials. This partnership will assist Pacylex in executing the steps necessary to file for regulatory authorization and to dose the first patient with PCLX-001 in 2020.
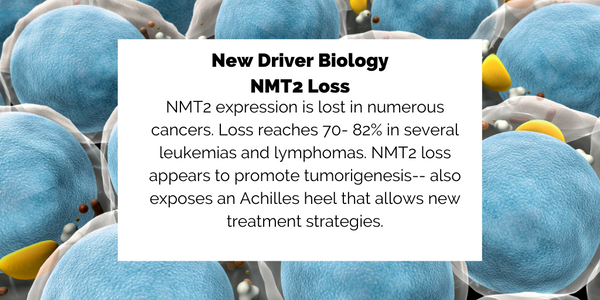
PCLX-001
Pacylex is developing an oral small molecule drug, PCLX-001, to treat cancers low in N-myristoyltransferase 2 (NMT2), a myristoylation enzyme whose deficiency is associated with higher mortality in leukemia and lymphoma. The company’s results to date indicate that PCLX-001 selectively kills cancer cells and completely regresses (eliminates) tumors in animal models of acute myeloid leukemia (AML), diffuse large B-cell lymphoma (DLBCL) and Burkitt’s lymphoma (BL). PCLX-001 has also been shown to inhibit the growth of lung and breast cancer tumors in animal models. In tests using cultured cancer cells in vitro, PCLX-001 is at least ten times as potent as Ibrutinib (Imbruvica) and Dasatinib (Sprycel), two drugs currently used for hematologic malignancies. Pacylex completed GLP toxicology dosing in January and expects to file for regulatory authorization to commence clinical studies in 2020.
About Pacylex
Pacylex is a pre-clinical pharmaceutical company targeting hematologic cancers with a new first-in-class therapeutic, and is headquartered in Edmonton, Alberta. Pacylex’s technology combines new insights from Dr. Luc Berthiaume of the University of Alberta, connecting myristoylation to cancer, with a family of high quality myristoylation inhibitors developed for African sleeping sickness by the University of Dundee Drug Discovery Unit in a program funded by Wellcome Trust. Pacylex licensed the molecules from the University of Dundee in 2015 to develop in oncology. PCLX-001 is the lead drug in a new class of NMT inhibitors, exploiting this new clinical target for cancer treatment. Pacylex plans to begin clinical studies of PCLX-001 in 2020 in diffuse large B-cell lymphoma and solid tumors. Pacylex is part of the Merck Invention Accelerator at TEC Edmonton and part of the current Creative Destruction Labs HealthWest 2019-2020 cohort.
About Davos
DavosPharma is a contract management organization engaged in pharmaceutical development for over 48 years. Through its established portfolio of strategic partners and internal expertise, DavosPharma has developed a broad scope of capabilities ranging from early discovery (medicinal chemistry, genomics, target validation, and assay development), through development operations (toxicology, pharmacology, formulation and API manufacturing) of small molecules and biologics. DavosPharma believes it is uniquely positioned to provide IND / NDA development support through its fully integrated translational turn-key approach that has enabled its pharmaceutical clients to achieve filings on a strict timeline. With biologists and chemists on staff, DavosPharma provides guidance and manufacturing capabilities for both small molecule (including cytotoxic and high potency) and biologics (antibodies, ADC’s, protein and peptide therapeutics). Formulation experience includes IV, Oral, Sub Q, IP, Ocular, Dermal, Intranasal and Inhalation routes of administration. DavosPharma offers CMC, toxicology and clinical regulatory support for writing and execution of IND’s and updates through NDA with the FDA. DavosPharma is responsible for the launch of over 20 commercial drugs.
Follow Pacylex on Twitter and LinkedIn
Click HERE for more information about Pacylex