Edmonton, Alberta, Canada, September 3, 2024. Pacylex Pharmaceuticals Inc. (Pacylex) is a clinical-stage pharmaceutical company that develops N-myristoyltransferase (NMT) inhibitors as targeted therapies for the treatment of hematologic cancers and solid tumors. Today, Pacylex announced that it is seeking partners to develop its large family of NMT inhibitors as a new class of Antibody Drug Conjugate (ADC) payloads. To introduce its ADC payload molecules, Pacylex will participate in the upcoming ADC & Radiopharmaceuticals Pharma & Biotech Partnering Summit on September 9-10 at the Revere Hotel Boston Common, Boston, Massachusetts, and the 15th World ADC Summit, November 4-7 at the Town & Country Resort in San Diego, California.
ADCs have emerged as a major category of therapeutics, particularly in the field of oncology. Despite this, only a very limited number of ADC payloads have been used to kill cancer cells. This new class of payload, NMT inhibitors, simultaneously affect multiple processes critical to cancer cell growth and survival and have been shown to regress solid tumor cancers in animal models when coupled with ADCs. Pacylex has exclusive license to 503 NMTis, 27 of which have single-digit nM ICs against human NMT1. The lead molecule in Pacylex’s NMTi collection, zelenirstat, a first-in-class myristoylation inhibitor, has been shown to inhibit the myristoylation required for assembly, translocation, and/or function of validated targets, such as B-cell receptor, Flt3-cKit complex, EGFR, and VEGFR. Zelenirstat also blocks respiratory Complex I formation in the mitochondria of cancer cells, thereby shutting down oxidative phosphorylation, which is critical for metastasis and cancer stem cells.
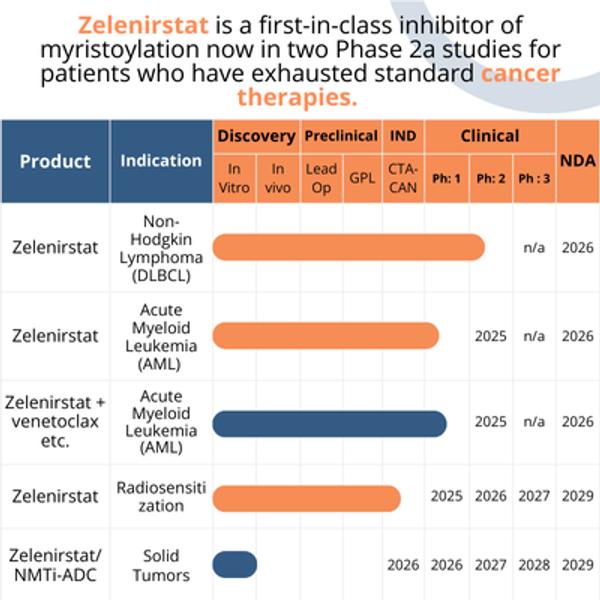
Zelenistat is the only clinically validated NMTi; it is well tolerated in Phase 1 patients with refractory/relapsed (r/r) lymphoma and refractory solid tumors. Phase 1 safety, PK, and drug exposure results with oral zelenirstat in 24 heavily pretreated NHL and solid tumor patients showed no dose-limiting toxicities. Patients receiving the recommended Phase 2 dose (RP2D) had significantly better progression-free and overall survival than those treated at lower doses; 57% had stable disease or better for six months or longer, despite cancers originating in 5 different organs. A patient with colorectal cancer who only achieved short-term benefits from the 5 prior lines of therapy, received the RP2D of zelenirstat for 475 days with reductions of approximately 50% in carcinoembryonic antigen (CEA) and tumor volumes. A Phase 2a patient with DLBCL has achieved a partial response and is on their 13th 28-day cycle of zelenirstat.
“The ability of NMTis to interrupt many critical cancer processes simultaneously makes them exciting drug candidates but also excellent potential ADC payloads”, said Dr. Michael Weickert, CEO of Pacylex. “Our collection of potent NMTis is one of only two families of molecules shown independently to be potent and on target, and zelenirstat is the only clinically validated NMTi. We want to establish ourselves as a partner of choice for those seeking novel and effective payloads for their ADC programs.”
For additional information, please visit www.pacylex.com.
Pacylex Pharmaceuticals Contact: Michael J. Weickert
CEO, Pacylex Pharmaceuticals, Inc.
E: michael.weickert@pacylex.com
P: 650-218-1840
Twitter @Pacylex (https://twitter.com/pacylex)
LinkedIn (www.linkedin.com/company/pacylex-pharma)
Facebook (https://www.facebook.com/pacylex)
About zelenirstat (aka PCLX-001 or DDD86481)
Zelenirstat (formerly identified as PCLX-001 or DDD86481) is a first-in-class, oral, small-molecule NMTi being developed to treat patients with leukemia, lymphoma, and for the treatment of solid tumors when used as a payload for ADCs. Zelenirstat selectively kills cancer cells in vitro and has been shown to regress hematologic malignancies and inhibit the growth of lung and breast cancer tumors in animal models.
Pacylex completed a Phase 1 multiple ascending dose safety, tolerability, and pharmacokinetics study on zelenirstat in patients with relapsed/refractory lymphoma and refractory solid tumors (NCT04836195). Zelenirstat demonstrated an acceptable safety and tolerability profile, pharmacokinetics consistent with once-daily oral dosing, and early signs of efficacy.
Zelenirstat is currently being studied in a Phase 2a open-label study designed to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical activity of zelenirstat in patients with relapsed/refractory B-cell non-Hodgkin Lymphoma (NHL) and in a separate Phase 1 / 2 study in AML patients is about to commence.
About NMTi inhibitors as ADC payloads
The effect of NMTis on cancer cells is pleiotropic and in different cancer types interrupts many pathways essential to proliferation and survival including: Src family kinases, Wnt pathway, PI3K/AKT/mTOR signaling pathway, Autophagy, Notch signaling, TLR4 inflammatory responses, AMPK, B-cell and T-cell receptor signaling pathway, Flt3/c-Kit signaling, Ferroptosis, ER-Golgi trafficking via ADP-ribosylation factors, VEGFR, EGFR, and mitochondrial respiratory Complex 1 formation. NMTi induces oxidative stress and apoptosis in cancer cells. The cancer cytotoxicity of NMTis makes them an exciting new class of ADC payload. In animal models, NMTi-ADCs have regressed solid cancer tumors.
Pacylex has exclusive rights to 503 small-molecule N-myristoyltransferase inhibitors (NMTis), including 27 with single-digit IC50 (<10nM) against Human NMT1. Zelenirstat has the following potential advantages as an NMTi-ADC payload: 1) clinical validation of safety, 2) clinical validation of anti-cancer activity, 3) multiple mechanisms of anti-cancer activity that disrupt multiple cancer signaling and survival pathways simultaneously and anticipated to work against cancers resistant to other payloads, and 4) limited resistance to long-term drug exposure due to plethoric pathways affected.
About Pacylex Pharmaceuticals
Pacylex is a clinical-stage pharmaceutical company headquartered in Edmonton, Alberta, Canada, which targets hematologic cancers with orally bioavailable NMT inhibitors and solid tumor cancers with NMTi used as ADC payloads. Zelenirstat is the lead drug in a new class of NMT inhibitors, enabling Pacylex to exploit NMTs as new clinical targets for cancer treatment and a new class of ADC payloads. Pacylex is conducting a multi-center Phase 2a studies in Canada in patients with relapsed/refractory NHL and the IND for a Phase 1 multiple ascending dose study in acute myeloid leukemia (AML) has been cleared, and the FDA has granted zelenirstat both Orphan Drug Designation and Fast Track Designation for AML. The US Department of Defense is supporting the initial clinical investigation of zelenirstat in AML patients. The Cure Cancer Foundation, Alberta Cancer Foundation, and Alberta Innovates supported the initial clinical studies.
Forward-Looking Statements
This press release contains forward-looking statements and forward-looking information within the meaning of applicable securities laws, such as statements relating to future events or the Company’s future financial and operating performance, as well as the Company’s business plans, growth initiatives, and objectives and prospects. Generally, forward-looking statements and forward-looking information may be identified by the use of forward-looking terminology, including the words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “goal,” “expect,” “strategy,” “future,” “likely,” "proposed,” “scheduled,” “forecast,” “budget,” “could,” “would,” variations of such words of phrases and other similar expressions, or by the use of words or phrases which state that certain actions, events or results may, could, would, or might occur or be achieved. However, the absence of these words does not mean that a statement is not forward looking. Forward-looking statements and forward-looking information are subject to numerous factors, risks and uncertainties that could cause actual results to differ materially, including, but not limited to, the Company’s ability to successfully execute on its business plans and strategies, avoid delays in planned clinical trials, hire and retain key personnel, obtain appropriate or necessary governmental approvals to market potential products and obtain future funding for product development and working capital on commercially reasonable terms, changes in laws, and general macroeconomic conditions, including economic slowdowns, recessionary risks, rising inflation and interest rates, and supply chain disruptions. Forward-looking statements and forward-looking information are based on the beliefs of management as well as assumptions made by and information currently available to management as of the date hereof, and none of the Company or its affiliates undertakes any obligation to update or issue revisions to any forward-looking statements or forward-looking information contained herein to reflect any future events or circumstances, except as required by law. The foregoing does not constitute an offer or solicitation to acquire any securities in the Company or any related or associated entity or affiliate. The information contained herein is not intended as legal, tax, financial or investment advice. Furthermore, the information contained herein may not be applicable to or suitable for an individual’s specific circumstances or needs.