Edmonton, Alberta, Canada October 10, 2024. Pacylex Pharmaceuticals Inc. (Pacylex) is a clinical-stage pharmaceutical company developing small-molecule N-myristoyltransferase inhibitors (NMTis) as targeted therapies for the treatment of hematologic cancers and as payloads for antibody drug conjugates (ADCs) for solid tumors treatment. Today, Pacylex announced the publication of a new study in Molecular Cancer Therapeutics, "Zelenirstat Inhibits N-Myristoyltransferases to Disrupt Src Family Kinase Signaling and Oxidative Phosphorylation Killing Acute Myeloid Leukemia Cells," describing how NMT inhibition by zelenirstat affects Acute Myeloid Leukemia (AML) cells, with a particular focus on describing the mechanism of action in the leukemia stem cell population.
To understand how zelenirstat kills AML cancer cells, researchers used AML patient-derived xenografts and AML cell line models to demonstrate that the disease-driving leukemia stem cell population is preferentially targeted. They also discovered that zelenirstat treatment disrupts SFK signalling and promotes apoptosis while also disrupting the mitochondrial complex I in AML mitochondria. Zelenirstat treatment results in the loss of the myristoylated mitochondrial complex I assembly factor NDUFAF4, complex I assembly and activity, oxidative phosphorylation, and respiration in AML cells. The study also demonstrated that the 54 gene myristoylation inhibition sensitivity signature, MISS-54, was highest in AML patients with the shortest life expectancy, suggesting that zelenirstat could address a large unmet medical need in the AML patient population.
“Our demonstrations of the ability of a myristoylation inhibitor to limit oxidative phosphorylation have now been exploited to preferentially target the leukemia stem cell population in AML models,” said Dr. Luc Berthiaume, CSO of Pacylex Pharmaceuticals. “Zelenirstat is a dual-action agent that inhibits cell signaling and oxidative phosphorylation, allowing it to effectively target both AML blasts and leukemic stem cells.”
“The selective activity of zelenirstat against the leukemia stem cell population in AML models holds great promise for AML patients, especially those with the worst outcomes,” said Dr. John Mackey, CMO of Pacylex Pharmaceuticals. “And the novel mechanism of action of our lead inhibitor shows exciting potential when combined with approved AML drugs in vitro.”
Pacylex Pharmaceuticals Contact: Michael J. Weickert
CEO, Pacylex Pharmaceuticals, Inc.
P: 650-218-1840
About zelenirstat (aka PCLX-001 or DDD86481)
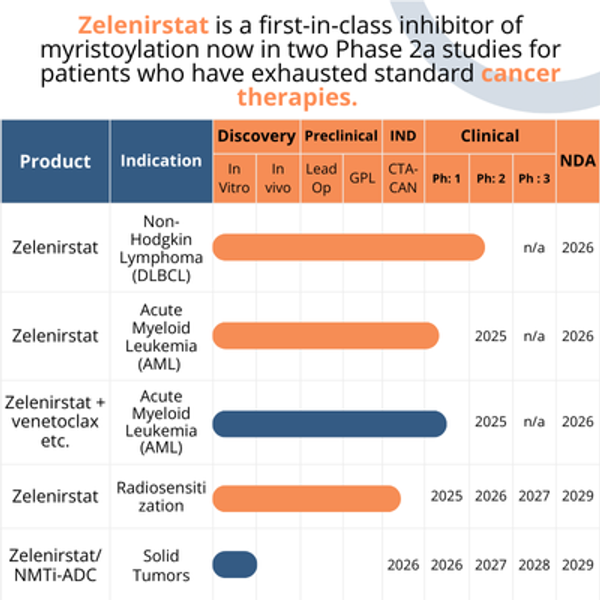
Zelenirstat (formerly identified as PCLX-001 or DDD86481) is a first-in-class, oral, small-molecule NMTi being developed to treat patients with leukemia, lymphoma, and for the treatment of solid tumors when used as a payload for ADCs. Zelenirstat selectively kills cancer cells in vitro and has been shown to regress hematologic malignancies and inhibit the growth of lung and breast cancer tumors in animal models.
Pacylex completed and published a
Phase 1 multiple ascending dose safety, tolerability, and pharmacokinetics study on zelenirstat in patients with relapsed/refractory lymphoma and refractory solid tumors (
NCT04836195). Zelenirstat demonstrated an acceptable safety and tolerability profile, pharmacokinetics consistent with once-daily oral dosing, and early signs of efficacy.
Zelenirstat is currently being studied in a Phase 2a open-label study designed to evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical activity in patients with relapsed/refractory B-cell non-Hodgkin Lymphoma (NHL) and in a separate Phase 1 / 2 study in AML patients is about to start.
About NMTis as ADC payloads
The effect of NMTis on cancer cells is pleiotropic. Across multiple cancer types, NMTis interfere with pathways essential to proliferation and survival including: Receptor tyrosine kinases (e.g. EGFR and VEGFR)/SFK pathways, Wnt pathway, PI3K/AKT/mTOR signaling pathway, Notch signaling, TLR4 inflammatory responses, B-cell and T-cell receptor signaling pathway, Flt3/c-Kit signaling, ferroptosis, ER-Golgi trafficking via ADP-ribosylation factors (Arfs), AMPK signaling, autophagy and mitochondrial respiratory complex 1 assembly and function. NMTis induce ER stress and apoptosis in cancer cells. The preferential toxicity of NMTis towards cancer cells makes them an exciting new class of ADC payload. In animal models, NMTi-ADCs have regressed solid cancer tumors.
Pacylex has exclusive rights to 503 small molecules NMTis, including 27 high potency compounds (IC50 <10nM) against human NMT1. Zelenirstat has advantages as an NMTi-ADC payload: 1) clinical validation of safety, 2) clinical validation of anti-cancer activity, 3) multiple mechanisms of anti-cancer activity that disrupt novel cancer signaling and survival pathways as well as metabolism, 4) activity in models of drug-resistant cancer, 5) limited acquired resistance despite long-term drug exposure. The investigation of the potential of zelenirstat and its analogs as ADC payloads has begun.
About Pacylex Pharmaceuticals
Pacylex is a clinical-stage pharmaceutical company headquartered in Edmonton, Alberta, Canada, which targets hematologic cancers with orally bioavailable NMTi zelenirstat and solid tumor cancers with NMTis used as ADC payloads. Zelenirstat is the lead drug in a new class of NMTis, enabling Pacylex to exploit NMTs as new clinical targets for cancer treatment and a new class of ADC payloads. Pacylex is conducting a multi-center Phase 2a studies in Canada in patients with relapsed/refractory NHL and the IND for a Phase 1 multiple ascending dose study in AML has been cleared, and the FDA has granted zelenirstat both Orphan Drug Designation and Fast Track Designation for AML. The US Department of Defense is supporting the initial clinical investigation of zelenirstat in AML patients. The Cure Cancer Foundation, Alberta Cancer Foundation, and Alberta Innovates supported the initial clinical studies. For additional information, please visit
www.pacylex.com.
Forward-Looking Statements
This press release contains forward-looking statements and forward-looking information within the meaning of applicable securities laws, such as statements relating to future events or the Company’s future financial and operating performance, as well as the Company’s business plans, growth initiatives, and objectives and prospects. Generally, forward-looking statements and forward-looking information may be identified by the use of forward-looking terminology, including the words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “goal,” “expect,” “strategy,” “future,” “likely,” "proposed,” “scheduled,” “forecast,” “budget,” “could,” “would,” variations of such words of phrases and other similar expressions, or by the use of words or phrases which state that certain actions, events or results may, could, would, or might occur or be achieved. However, the absence of these words does not mean that a statement is not forward looking. Forward-looking statements and forward-looking information are subject to numerous factors, risks and uncertainties that could cause actual results to differ materially, including, but not limited to, the Company’s ability to successfully execute on its business plans and strategies, avoid delays in planned clinical trials, hire and retain key personnel, obtain appropriate or necessary governmental approvals to market potential products and obtain future funding for product development and working capital on commercially reasonable terms, changes in laws, and general macroeconomic conditions, including economic slowdowns, recessionary risks, rising inflation and interest rates, and supply chain disruptions. Forward-looking statements and forward-looking information are based on the beliefs of management as well as assumptions made by and information currently available to management as of the date hereof, and none of the Company or its affiliates undertakes any obligation to update or issue revisions to any forward-looking statements or forward-looking information contained herein to reflect any future events or circumstances, except as required by law. The foregoing does not constitute an offer or solicitation to acquire any securities in the Company or any related or associated entity or affiliate. The information contained herein is not intended as legal, tax, financial or investment advice. Furthermore, the information contained herein may not be applicable to or suitable for an individual’s specific circumstances or needs.